Ultra Rapid Generation of (1H-Indol-3-yl)methyl Electrophiles and Their Use for Nucleophilic Substitution
A big data analysis published in 2016 revealed that heteroatom alkylations and arylations have been the most frequently used class of organic transformations in the last 40 years. Indole has been recognized as a privileged structure, ranking 13th most frequently used among the 351 ring systems found in marketed drugs. The heteroatom alkylation at the carbon atom next to the indole ring is potentially useful for producing a variety of indole derivatives. However, electron-rich indole tends to occur undesired reactions very rapidly, therefore, such transformation have been hampered. We successfully performed ultrarapid generation (20 ms) of (1H-indol-3-yl)methyl electrophiles in a micro-flow reactor and their rapid use (100 ms) for subsequent hetroatom alkylation. No desired product was obtained when this reaction was carried out under batch conditions.
Commun. Chem. 6, 47, (2023).
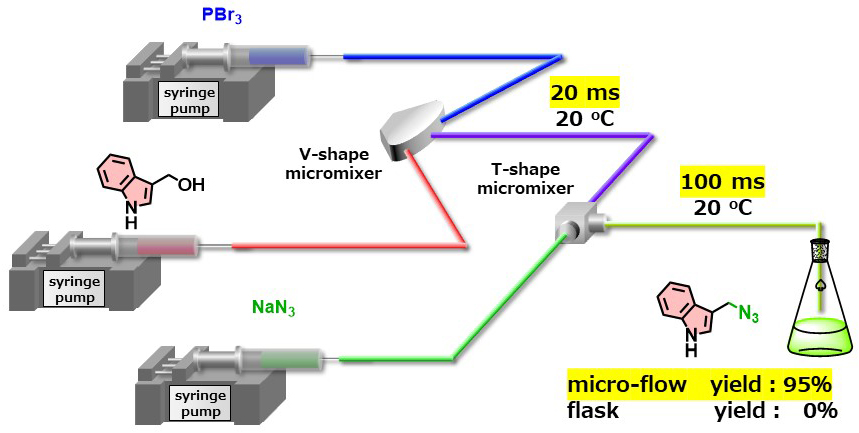
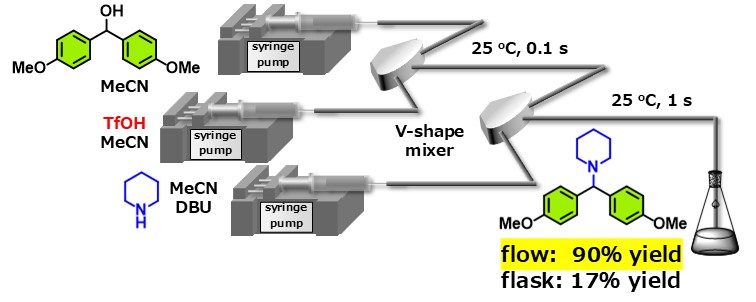
Ultra Rapid Generation of Carbocations and Their Use for Nucleophilic Substitution
Acid-mediated generation of carbocations from alcohols and subsequent nucleophilic substitutions is one of the most fundamental transformations in synthetic organic chemistry. However, because acids are used to activate the alcohols, it is challenging to use nucleophiles with high basicity, such as amines, in these transformations. This is due to the rapid protonation and subsequent deactivation of the amines by the acids. We have successfully utilized the advantages of microflow synthesis to activate alcohols with strong acids, TfOH, within 0.1 seconds and the following reaction of the generated carbocations with amines within 1 second, achieving high yields of the desired products. When this reaction was conducted using a flask, multiple side reactions competed, resulting in a yield of only 17% for the target product.
Chem. Commun. 60, 2497, (2024).
We have also reported other rapid microflow nucleophilic substitution reactions on carbons adjacent to aromatic rings!
Microflow rapid nucleophilic substitution reaction on the carbon adjacent to the 2-position of indole
Synthesis, 56, (17), 2663-2669, (2024).
Microflow rapid nucleophilic substitution reaction on the carbon adjacent to the furan ring
Org. Biomol. Chem. 22, (17), 3448-3452, (2024).
Microflow rapid nucleophilic addition-substitution sequential reaction on the carbon adjacent to the 3-position of indole
Chem. Asian J. 19, (1), e202300909, (2024).