Synthetic chemists have used flasks to carry out reactions. At least, several seconds are required to complete a mixing of solutions in a flask, even under vigorous stirring conditions. Therefore, the precise control of short reaction time (< ca. 60 sec) is very difficult using flasks.
Micro-flow synthesis uses thin channels (inner diameter < ca. 1mm) to carry out reactions. Rapid mixing (< several milli seconds) can be readily performed using the micro-flow synthesis. For instance, we can obtain a rapidly mixed solution (light blue) from outlet of a T-shape mixer shown below by injecting two solutions (red and blue) into inlets of the T-shape mixer at high flow rates. In addition, temperature of the solution can be precisely controlled under micro-flow conditions due to large surface-to-volume ratio compared with that of batch conditions (flask).
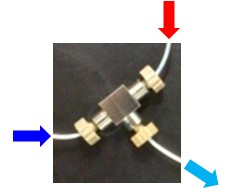
There are many organic transformations that occur side reactions without precisely controlling the short reaction time (< 60 sec). These transformations have been considered to be useless in synthetic organic chemistry. We have tried to create values of these reactions by the use of micro-flow technology.