Syntheses of Peptides
Peptides have been increasingly important as drugs in last several decades. Development of efficient synthetic processes for peptides remains a highly important pursuit. Because traditional batch peptide syntheses require the use of excess amount of expensive and wasteful reagents with mild electrophilicity to suppress undesired reactions. We developed micro-flow peptide bond formation using inexpensive and less wasteful reagents with high electrophilicty. The key to success was avoidance of side reactions via precise control of reaction time and temperature by the use of micro-flow technology.
Angew. Chem. Int. Ed. 53, (3), 851-855 (2014).
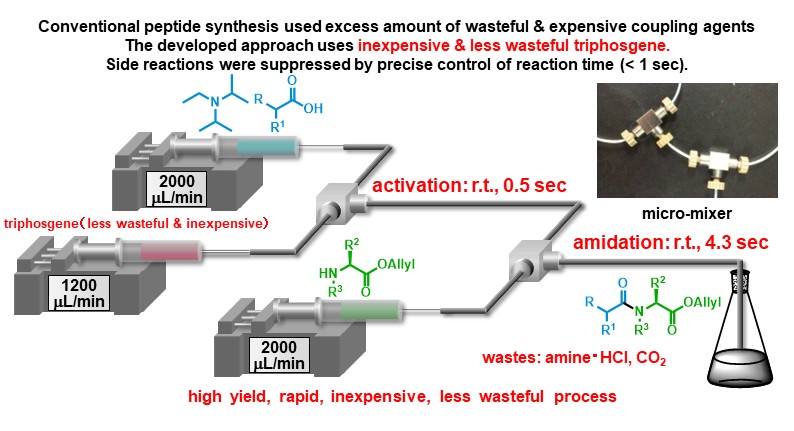
We achieved total synthesis of feglymycin via the developed micro-flow peptide bond formation. The total synthesis was achieved via one by one peptide chain elongation that was thought to be impossible due to competitive racemizations of substituted phenyl glycines.
Nat. Commun. 7, 13491 (2016).
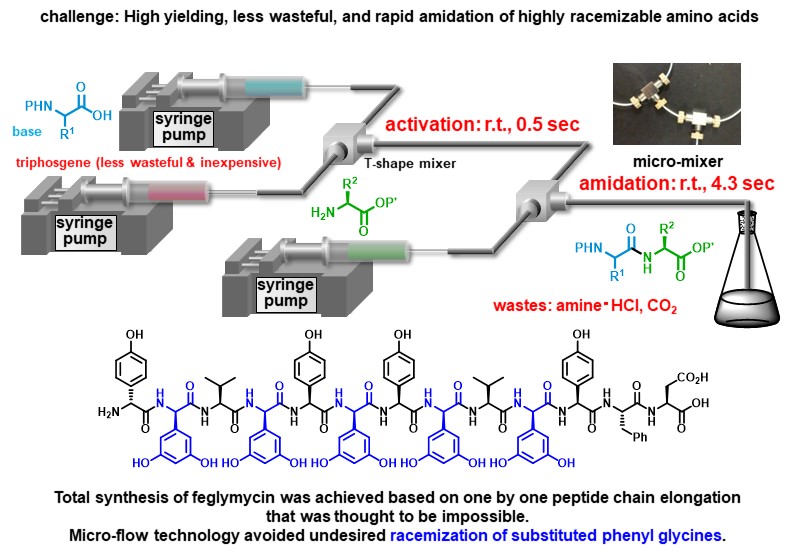
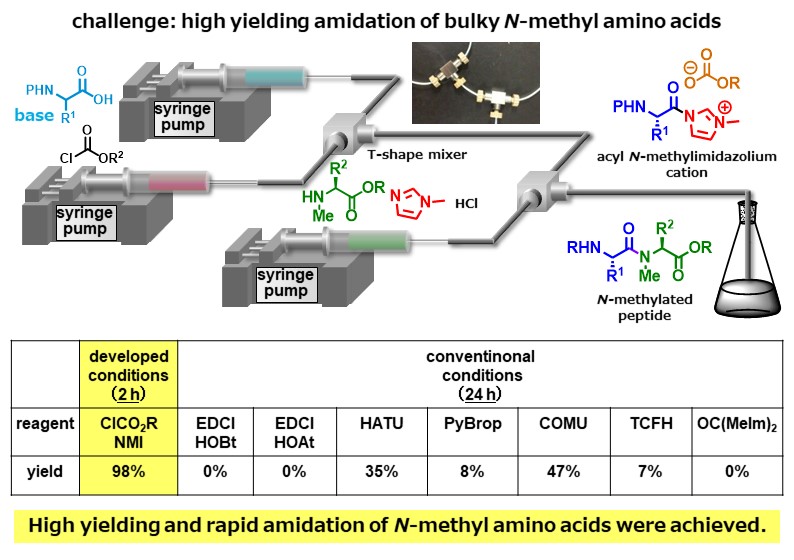
N-Methylated peptides have garnered much attention as drugs and drug candidates, however, high-yielding peptide bond formation using sterically hindered N-methyl amino acids remains a challenging task. We overcame this problem by using highly electrophilic acyl N-methylimidazolium cation species. In addition, we observed that the addition of HCl dramatically accelerated the reaction. This is a previously unknown phenomenon. The developed micro-flow peptide bond formation allowed us to obtain a variety of N-methylated peptides in higher yields in shorter times compared with the previously reported conditions.
Angew. Chem. Int. Ed. 59, (31), 12925-12930 (2020).
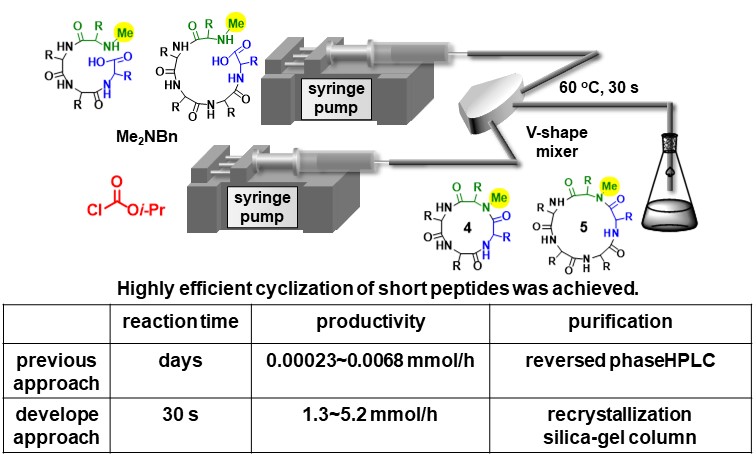
Cyclic N-methylated peptides have become increasingly important as drugs because they usually have better metabolic stability, selectivity and affinity against their biological targets. In addition, they sometime enable cell membrane permeation and oral administration. However, cyclization of peptides usually require the use of a large amount of expensive coupling agents and long reaction time (days). In particular, cyclization of short peptides (<6 residues)are generally difficult. We developed high yielding, low cost, and rapid peptide cyclization of the short peptides using highly active acyl ammonium ion. The developed approach dramatically shorten reaction time. In addition, obtained peptides can be readily purified. Productivity of the aimed cyclic peptide was improved up to 22,600 folds.
Angew. Chem. Int. Ed. 62, (27), e20230647, (2023).
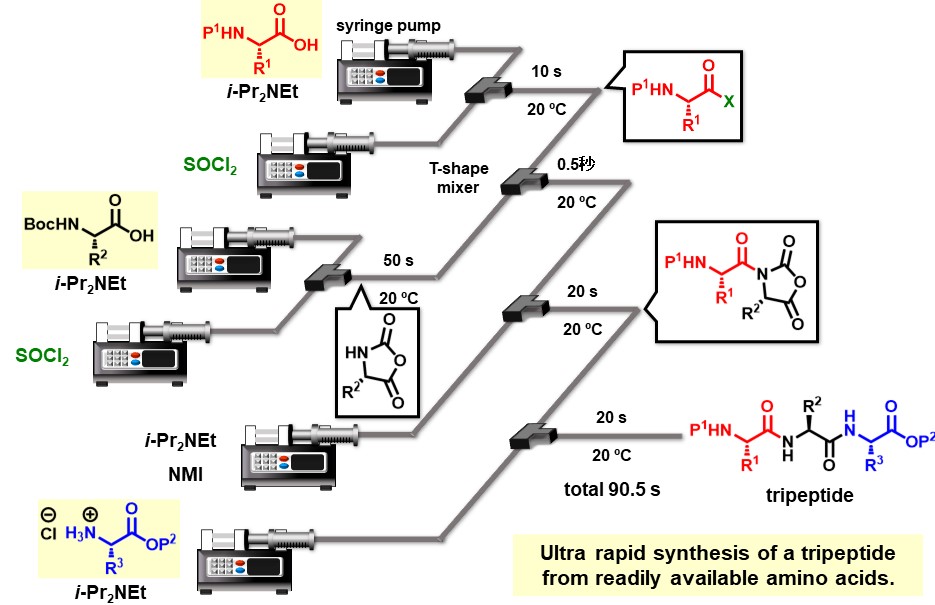
Peptide chain elongation repeats coupling of protected amino acids and deprotection steps. Therefore, half of all the synthetic steps is deprotection steps. Sequential coupling of amino acids without the deprotection steps enable rapid and low cost production of peptides. We achieved ultrafast peptide chain elongation via one-flow three-component coupling approach using NCAs described herein after.
Chem. Sci. 14, (25), 6986-6991 (2023).
Synthesis of Amino Acid Derivatives
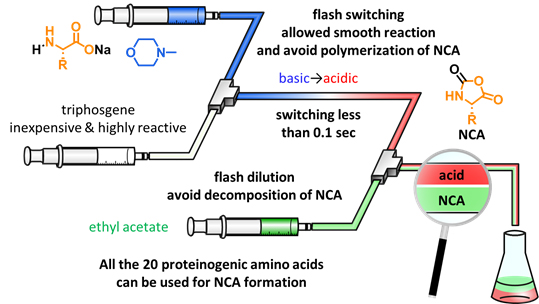
Amino acid N-carboxyanhydrides (NCAs) are frequently used as monomers for polypeptide synthesis. The most conventional synthetic approach for NCAs that was reported about 100 years before includes the use of strong acidic conditions. Therefore, synthesis of NCAs containing acid-labile functional groups have been difficult. On the other hand, the desired NCAs can be generated rapidly under basic conditions, but they spontaneously occur oligomerization/polymerization under the basic conditions. We successfully solved these problems by developing basic-to-acidic flash switching technology. The desired NCAs were rapidly generated under basic conditions, then pH was immediately changed by internally generated HCl that avoided the undesired oligomerization/polymerization. A variety of NCAs containing acid-labile functional groups were prepared in high yields.
Angew. Chem. Int. Ed. 57, (35), 11389-11393 (2018).
We have also reported many other microflow syntheses of peptides and amino acid derivatives!
Peptoid chain elongation while suppressing undesired intramolecular cyclization using microflow technology
Org. Lett. 26, (48), 10280-10284 (2024).
Microflow synthesis of C-terminal free N-methylated peptides. Verification of the activation effect of acid anhydrides by Brønsted acids and epimerization of C-terminal free N-methylated peptides
Chem. Eur. J.30, (38), e202401402, (2024).
Microflow synthesis and utilization of BocCl equivalents. Microflow synthesis of a Boc-protected α-amino acid N-carboxyanhydride was also reported
Org. Process Res. Dev. 28, (5), 1971-1978 (2024).
One-flow synthesis of cyclic N-methylated peptides via sequential coupling-cyclization of C,N-terminal free dipeptides
Eur. J. Org. Chem. 26, (35), e202300700, (2023).
Microflow synthesis of urethane-protected α-amino acid N-carboxyanhydrides. Stability of acylammonium ions and α-amino acid N-carboxyanhydrides also reported
Org. Biomol. Chem. 20, (16), 3303-3310, (2022).
Microflow peptide cyclization using triphosgene
Chem. Eur. J. 27, (27), 7525-7532 (2021).
Rapid dual activation in a microflow reactor for the synthesis of β-amino acid derivatives
Chem. Commun. 56, 4527-4530 (2020).
Microflow synthesis of β-amino acid N-carboxyanhydrides
Chem. Asian J. 15 (1), 79-84, (2020).
Microflow synthesis of C-terminal free peptides using C,N-terminal free amino acids
Chem. Eur. J. 25, (66), 15091-15097, (2019).
Peptide cyclization using photochemical microflow reaction
Org. Biomol. Chem. 14, (47), 11244-11249, (2016).
Synthesis of 3,5-dihydroxyphenylglycine using photochemical microflow reactions
J. Flow. Chem. 4, (4), 173-179, (2014).
Microflow amide bond formation using in situ generated phosgene
Chem. Commun. (47), 12661-12663, (2011).