Synthesis of Phosphotriesters
Phosphotriesters are important as intermediates in oligo nucleotide synthesis, prodrugs, drug candidates, pesticides, flame retardants, and plasticizers. Almost all the organophosphorus compounds have been synthesized from inexpensive and readily available phosphorus trichloride. However, it was difficult to synthesize phosphotriesters via sequential introduction of three different alcohols against phosphorus trichloride because over nucleophilic attack of alcohols readily occurs due to high electrophilicity of phosphorous trichloride. Interestingly, the risk of overnucleophilic attack during introduction of the second alcohol is higher than that during introduction of the first alcohol. The addition of imidazole suppress the undesired overnucleophilic attack during introduction of the second alcohol. However, although these are fundamental issues in organophosphorus chemistry, detailed explanations were offered neither for the decreased selectivity in the absence of imidazole and nor for the improved selectivity in the presence of imidazole. We developed micro-flow synthetic approach that enabled rapid synthesis of phosphotriesters via sequential introduction of three different alcohols against phosphorus trichloride. We also proposed reasons for the decreased selectivity in the absence of imidazole and for the improved selectivity in the presence of imidazole based on DFT calculation. This is a collaborative work with Tokyo Tech and Kanazawa university.
Chem. Eur. J. 28, (37), e202200932, (2022).

Switchable Acylation of H-Phosphonates
H-Phosphonates have been used as intermediates in the synthesis of various useful organic phosphorus compounds, however, examples of their acylation reactions have been limited. We have developed switchable acylation using acyl ammonium ion or acyl pyridinium ion that was prepared from the reaction of chloroalkyl formates with dimethylethylamine or 9-azajulolidine. Acyl ammonium ion induced O-acylation and subsequent decarboxylation and oxidation afforded phosphotriesters. This unique transformation was first discovered in this study. On the other hand, acyl pyridinium ion induced P-acylation affording phosphonoformate esters.
Chem. Commun. advance article
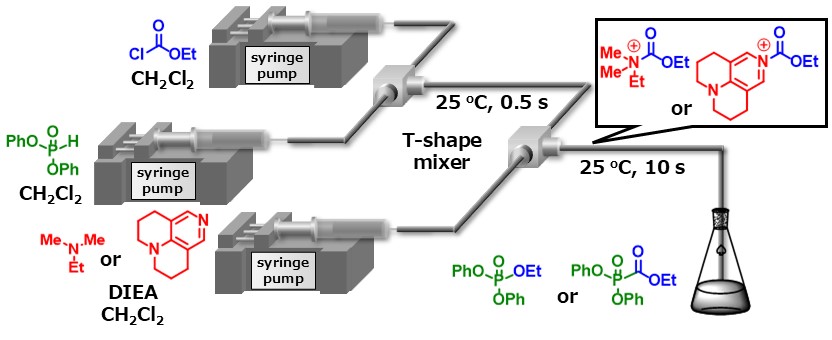
We have also reported other microflow synthesis of organophosphorus compounds!
Microflow synthesis of cyclic phosphotriesters
Chem. Asian J. 19, (11), e202400256, (2024).
Microflow synthesis of asymmetric H-phosphonates
J. Org. Chem. 89, (3), 1777-1783, (2024).